-
 Instant mass screeningfor carbon monoxide poisoning
Instant mass screeningfor carbon monoxide poisoning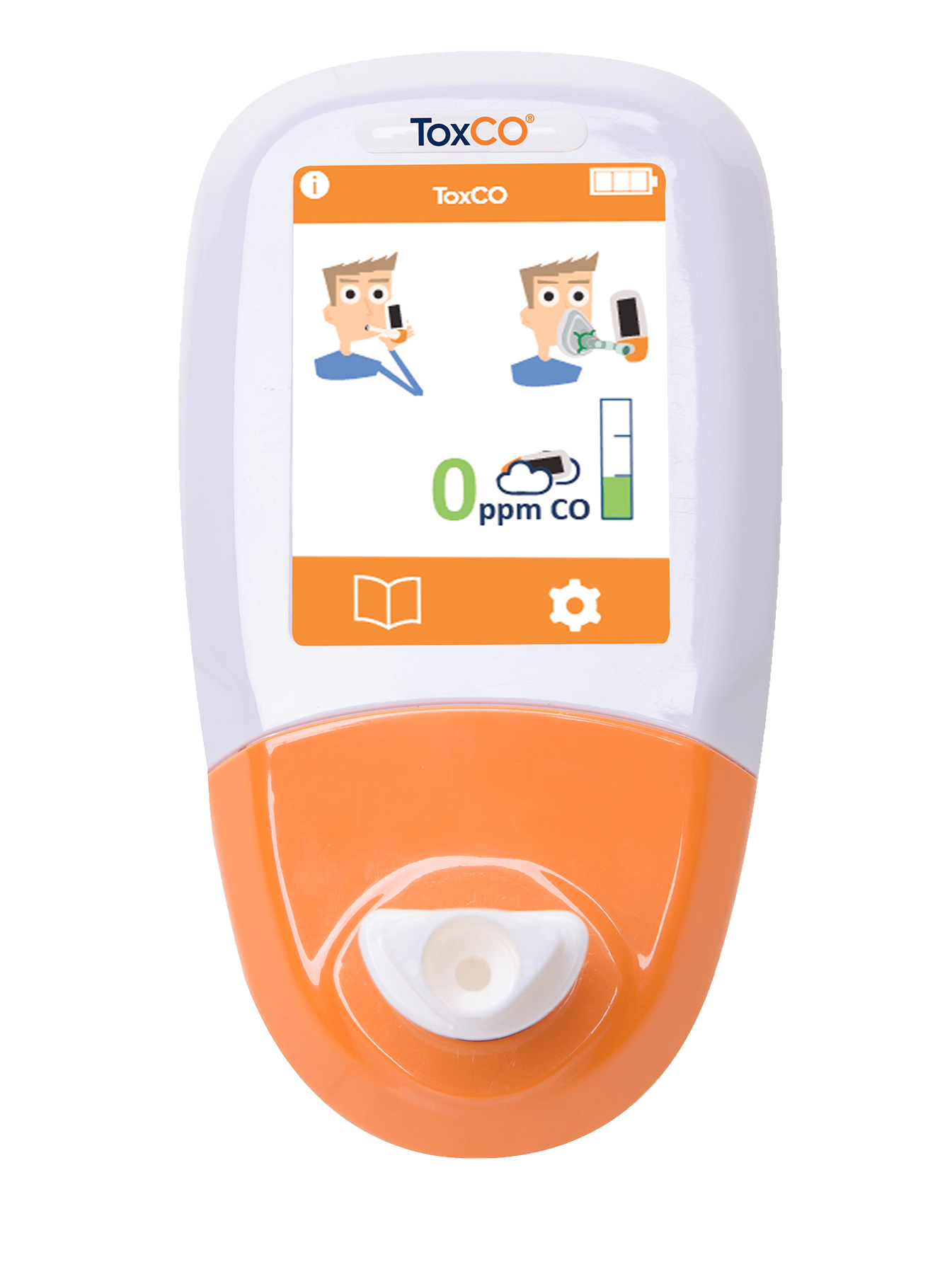
-


Welcome to the ToxCO® Device Website
About CO Poisoning
Carbon Monoxide (CO), known as the silent killer, is a tasteless, colourless, odourless gas, which is produced when things burn incompletely. Everybody is exposed to CO in everyday life; from boilers, open fires, cars, generators and even burning your toast.
CO binds 200 times more readily with your red blood cells to form carboxyhaemoglobin1. When you inhale CO, it replaces the oxygen in your bloodstream, preventing red blood cells from carrying oxygen to your organs. Because of this, prolonged or high levels of exposure can lead to severe health consequences, and in worse cases, death. An exposure to 1200ppm of carbon monoxide can kill you within 3 minutes2.
One of the significant problems with diagnosing CO poisoning is that it can engender similar symptoms to flu3, including headaches, nausea, vomiting, fatigue, and dizziness, however prolonged exposure results in physical, psychological and neurological damage.
Carboxyhaemoglobin levels can be determined by a blood test, a breath test, or CO-pulse oximetry.
How the ToxCO® device works
The ToxCO® is a breath carbon monoxide device, which instantly determines the level of carbon monoxide in your blood by measuring the parts per million (ppm) of CO exhaled on your breath. Measuring exhaled carbon monoxide levels offers a quick, reliable and non-invasive method to determine a patient’s carboxyhaemoglobin levels, and it is recommended by both NICE and PHE4,5.
The mouthpiece and face mask sampling modes allow the user to test a patient independent of their consciousness, whilst the ambient sampling mode safeguards the user, alerting them to high levels of CO in the atmosphere.
The ToxCO® can aid emergency personnel in quickly identifying if hospital admissions are necessary through its ability to instantly mass screen patient for CO poisoning, helping to save valuable time.
Features and Benefits
Revolutionary Interface
Full-colour touchscreen with easy-to-use
interface
3 Sampling options
Breath testing by mouthpiece or facemask to determine
levels of CO independent of a patient’s consciousness
Ambient mode to safeguard users levels of CO independent of a patient’s consciousness

Ergonomic Design
An ergonomic design, fully portable and
incorporated with SteriTouch® technology for
optimum infection control
Test Log
Automatically logs up to 500 readings
All results can be ‘tagged’ with a Name,
Location or ID for later reference
As easy as…


Technical Specification

Concentration range
0-54%COHb/0-600ppm

Repeatability
≤±5% difference on consecutive readings

Display
Full-colour touchscreen

Accuracy
≤±3ppm/10%
whichever is greater*

Detection principle
Electrochemical sensor

Storage/transport temperature
0-50°C

Response time
<30 seconds

Operating humidity
15-90%
non-condensing

Operating temperature
0-50°C
Power
3 x AA batteries
(Up to 1000 minutes)

Sensor operating life
2 years

H2 cross interference
≤6%
*±5ppb of measured value ≤50ppb or ±10% of measured value >50ppb
Consumables
Our consumables are made to the highest quality at the most cost effective prices.

Steribreath™ Eco Mouthpiece
SteriBreath™ Eco Mouthpieces are individually sealed for optimum infection control.
Condensation confirms an accurate breath sample. SteriBreath™ Eco Mouthpieces are attached to the device via the D-piece™.
SteriBreath™ Eco Mouthpieces are individually sealed for optimum infection control.
Condensation confirms an accurate breath sample. SteriBreath™ Eco Mouthpieces are attached to the device via the D-piece™.

D-piece™
The D-piece™ incorporates a one-way valve and an infection control filter, which removes and trap >99% of airborne bacteria and >97% of viruses6. The D-piece™ should be changed every four weeks.
The D-piece™ incorporates a one-way valve and an infection control filter, which removes and trap >99% of airborne bacteria and >97% of viruses6. The D-piece™ should be changed every four weeks.

Facemask Adaptor
The facemask adaptor is single-patient use. A facemask is attached to one end and then the sampling system connects to the device via the D-piece™.
The facemask adaptor is single-patient use. A facemask is attached to one end and then the sampling system connects to the device via the D-piece™.
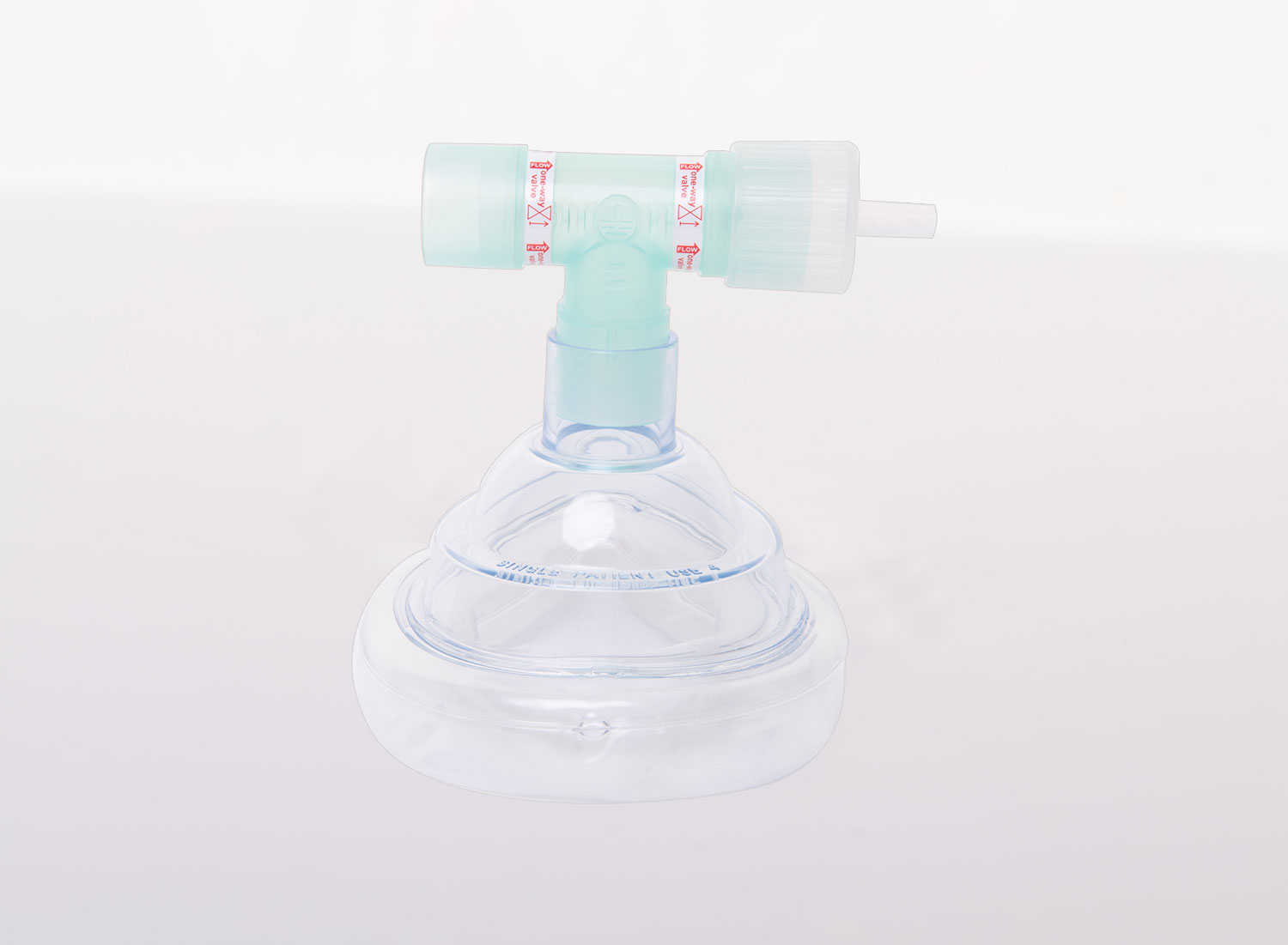
Facemask
Facemasks are single-patient use and allow the patient to breathe normally to produce a breath sample. There are 3 sizes: Large, Medium, and Small.
Facemasks are single-patient use and allow the patient to breathe normally to produce a breath sample. There are 3 sizes: Large, Medium, and Small.

Calibration Gas
The ToxCO® requires calibration every 6 months using 50ppm CO gas, provided as a kit or replacement cylinder.
The ToxCO® requires calibration every 6 months using 50ppm CO gas, provided as a kit or replacement cylinder.
Accreditations
We take the utmost pride in the fact that the NObreath is accredited to BS EN ISO13485:2016 and CE marked to the Medical Device Directive (MDD).


News
All the latest news on the ToxCO® device…

Breath Analysis to Screen for Carbon Monoxide Poisoning with the ToxCO®
Carbon monoxide (CO) poisoning is the most common cause of fatal accidental poisoning in the world. However, as it is... read more →
February 15, 2021
ToxCO® Device By Bedfont To Determine CO Poisoning Receives FDA Clearance
Bedfont’s ToxCO® device to determine CO poisoning receives FDA clearance The ToxCO® is a breath analysis device that measures exhaled carbon... read more →
August 28, 2019
Where to buy?
We operate through a network of carefully selected distributors worldwide – to find your
nearest distributor, click here
nearest distributor, click here
Medical Advisory Board
We work with a network of KOL’s in the medical world to make sure the ToxCO® device is fit for purpose. We are always working to improve our ToxCO® range and if you would like to be a part of this elite group of healthcare professionals, please contact Bedfont® today.
Our Mission
To save time through the rapid detection of CO poisoning through breath testing.
Our Purpose
To highlight the benefits of rapid CO breath testing in emergencies and make CO breath testing more accessible globally through an effective yet cost-efficient range of breath devices and equally low cost consumables.
Customer Charter
What we do:
- Work hard to design and manufacture the most up to date, high quality, innovative products for use by worldwide health professionals.
- Re-invest our profit into future research and development to keep our products up to life saving standards.
- Strive to produce high quality consumables at the lowest possible prices.
- Are committed to provide a very high level of quality customer service that takes account of the changing needs and expectations of our customers.
- Work to continuously improve the quality in all that we do.
References
- Blumenthal I. Carbon monoxide poisoning [Internet]. PubMed Central (PMC). 2001 [cited 25 July 2019]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1281520/
- CO Health Risks – Detect Carbon Monoxide [Internet]. Detect Carbon Monoxide. 2007 [cited 25 July 2019]. Available from: http://www.detectcarbonmonoxide.com/co-health-risks/
- Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emergency Medicine Journal. 2002;19(5):386-390.
- Scenario: Management of carbon monoxide poisoning [Internet]. Carbon monoxide poisoning – NICE. 2018 [cited 5 August 2019]. Available from: https://cks.nice.org.uk/carbon-monoxide-poisoning#!scenario
- Public Health England. Diagnosing Poisoning: Carbon Monoxide (CO). Public Health England; 2015.
- Public Health England. An evaluation of filtration efficiencies against bacterial and viral aerosol challengers report No. 17/001. Salisbury: Public Health England; 2017.
Literature
All of our literature can be found on the Bedfont® Scientific Ltd. website. We have a wide range of resources in a variety of languages, see here.
The ToxCO® device is a product from Bedfont® Scientific Ltd.
The ToxCO® is intended to aid in the diagnosis of suspected carbon monoxide poisoning and should be used alongside additional means to evaluate clinical signs and symptoms.
Bedfont® Scientific Ltd., Station Yard, Station Road, Harrietsham, Maidstone, Kent, ME17 1JA, England
© 2022 Bedfont® Scientific Ltd., all rights reserved | Privacy Policy | Bedfont Scientific Limited reserves the right to change or update this website without prior notice.
Incorporated in England and Wales under registered number: 1289798
© 2022 Bedfont® Scientific Ltd., all rights reserved | Privacy Policy | Bedfont Scientific Limited reserves the right to change or update this website without prior notice.
Incorporated in England and Wales under registered number: 1289798
![]()
Emergo Europe B.V.
Westervoortsedijk 60
6827 AT Arnhem
The Netherlands.







